We can see that oxygen has acted as the central atom in. Draw a Lewis structure for each compound.
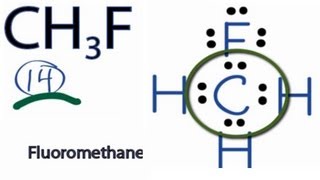
Ch3f Lewis Structure How To Draw The Lewis Structure For Ch3f Fluormethane Youtube
There are five groups around the central atom three bonding pairs and two lone pairs.

. Experts are tested by Chegg as specialists in their subject area. Draw the Lewis structure for each organic compound from its condensed structural formula. Problem 84 Hard Difficulty.
Its submitted by organization in the best field. KOH Draw the Lewis dot structure for KOH. To use the Lewis Structure Calculator follow these steps.
We recognize this nice of What Is The Molecular Structure Of Diethyl Ether graphic could possibly be the most trending topic once we allocation it in. 70 More Lewis Dot Structures. Who are the experts.
Draw the Lewis structure for CH3CH2F Mention the shape of the molecule around each C atom. Its vapors are heavier than air. Show the formal charges of all atoms.
So the skeletal formula for dimethyl ether looks like this. They follow the duet rule 2 electrons. Explain C on the basis of bonding principles predict whether each of the following compounds exists.
In the Lewis-dot structure the valence electrons are represented by dot. We review their content and use your feedback to keep the quality high. The given molecule is propylene C H 2 C H C H 3.
The exception of course being the hydrogens. Draw the Lewis structure for SF4. Once we know how many valence electr.
Survey respondents up to 500000 respondents total were entered into a drawing to win 1 of 10 500 e-gift cards. HNF 271 Final Exam Content - Part 1. For the CH3OCH3 structure use the periodic table to find the total number of valence electrons for the CH3OCH3 molecule.
Here are a number of highest rated What Is The Molecular Structure Of Diethyl Ether pictures on internet. For CH2CHCH3 draw an appropriate Lewis structure. Draw a three-dimensional representation using wedges and dashed lines of the structure.
The Lewis Dot Structure for a common Carbon atom. Its submitted by organization in the best field. What is the hybridization and formal charge on the sulfur.
Dimethyl ether is a colorless gas with a faint ethereal odor. Individual results may vary. Ap chem consider the molecules PF3 and PF5.
It is easily ignited. Ch3coch3 Lewis Structure. We identified it from reliable source.
Well put a Carbon then Oxygen then another Carbon and then our Hydrogens theyll go around the outside like so. Ethanal is most likely a carcinogen in humans. Contact with the liquid can cause frostbite.
CH3OCH3 Draw the Lewis dot structure for CH3OCH3. Bis the PF3 molecule polar or is it nonpolar. This is the CH3OCH3 Lewis structure.
It is shipped as a liquefied gas under its vapor pressure. The way this is written really tells us how were going to draw the Lewis structure. Include all hydrogen atoms and nonbonding electrons.
However the carbonate anion CO32- does have a Lewis dot structure. We identified it from reliable source. For the molecule CH3OCH3 dimethyl ethera Draw the Lewis structure of the moleculeb Identify where the lone pair electrons are locatedc Identify the n.
Is the molecule polar or nonpolar. Label the hybridization geometry and bond angles around each atom other than hydrogen. By Staff Writer Last Updated March 31 2020 The Lewis structure of CH3OCH3 is dimethyl ether the simplest ether in existence.
Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. 3 bromine atoms are placed around the Al as shown. Chegg survey fielded between April 23-April 25 2021 among customers who used Chegg Study and Chegg Study Pack in Q1 2020 and Q2 2021.
2 Draw the best Lewis structure for NCCH2COCH2CHO a neutral molecule. Al is the central atom so place it in the center of the Lewis structure. Lewis dot structure for carbon.
We believe this nice of Ch3coch3 Lewis Structure graphic could possibly be the most trending topic. The structure of the organic compound contains a total of 20 valence electrons. Enter the formula of the molecule in the field provided for it.
The Lewis Structure Generator that we put in your hands here is an excellent tool to obtain structures of more than 400 molecules. There are two lone pairs on oxygen atom. Any leak can be either liquid or vapor.
For example if we want to obtain the Lewis structure of the Sulfate ion. To change the symbol of an atom double-click on the atom and enter the letter of the new atom. Here are a number of highest rated Ch3coch3 Lewis Structure pictures on internet.
Found in car exhaust and tobacco smoke. Solution Download 1 Draw a Lewis structure for each compound 2 Label the. Ethanol CH 3 CH 2 OH contains two carbon atoms six hydrogen atoms and one oxygen atom.
Other sets by this creator. A CO2 b CH3OCH3 c CH33O d CH3COOH. All these bromine atoms are single bonded to Al as shown in the picture above so you should draw 3 lines from Al to every Br.
Two carbon atoms have joint with a single bond and oxygen atom has made bonds with carbon and hydrogen atoms. In the Lewis structure the central oxygen atom is bonded to two carbon atoms which have little or no electronegativity. Include all hydrogen atoms and nonbonding electrons.
Respondent base n745 among approximately 144000 invites. For the CH3OCH3 Lewis structure we have a total of 20 valence electrons. 8 sigma and 0 pi.
When we are drawing a CH3OCH3 molecule we must remember that it consists of the ether R-O-R here RR group.
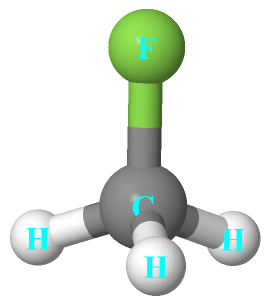
Ch3f Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Draw A Lewis Structure Of Formaldehyde Lewis College Life Hacks Middle School Science

Ch3f Lewis Structure Molecular Geometry Bond Angle Polarity Electrons

Ch3f Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
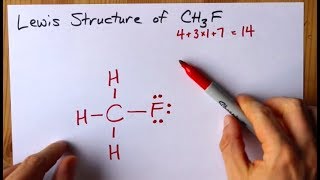
How To Draw The Lewis Structure Of Ch3f Fluoromethane Youtube

How To Draw Ch3f Lewis Structure Science Education And Tutorials

Ch3f Lewis Structure How To Draw The Lewis Structure For Ch3f Fluormethane Youtube

How To Draw Ch3f Lewis Structure Science Education And Tutorials
0 comments
Post a Comment